The Most Valued Use Case in Clinical Trials for Blockchain
From the moment technology professionals began assessing Use Cases for the Blockchain in Life Sciences, Clinical Trials have been at the top of the list. In an effort to make clinical studies more efficient from a time and cost perspective, the Blockchain has been identified as a solution in several Use Cases specifically pertaining to patient and investigator enrollment. Perhaps the high costs (2.6 billion USD – Average Cost) to develop a new drug or the low FDA approval rate (approximately 14%) has put so much emphasis and pressure on the Sponsor to expedite and deliver a successful product, that researchers have begun to closely examine the areas within the trial that have greatly impacted the time and cost factors to either start or complete a clinical study.
As Solution Providers have been called upon to determine whether the Blockchain can actually deliver a product that provides value for clinical trials, most solution providers have placed the focus in the areas of enrollment as this is been the sore spot for the Sponsor, Investigators, and Sites involved in a trial. As the goal has always been first patient in; last patient in and lock the database; there is much more to it than just applying a Blockchain for the validation and verification of patient and trial data. As a Blockchain can easily handle the management of data from the different sites; the challenge will always remain about the quality and integrity of the data. In addition, all the different sites must be Trusted Participants and/or Partners of a Blockchain or Permissioned Ledger to truly have high-quality data. Moreover, technology solution providers who do not have a deep understanding of the clinical trial process will continually mention the same Use Cases for enrollment as if that is the only Use Case within a trial that the Blockchain can support.
Because iSolve not only has a deep understanding of innovative technologies such as Blockchain and Artificial Intelligence, iSolve also has a team dedicated to developing solutions within all four phases of the clinical trial process. Which is why in the upcoming release of ADLT™, the clinical trial module will support the most valued Use Case in clinical trials: Study Startup.
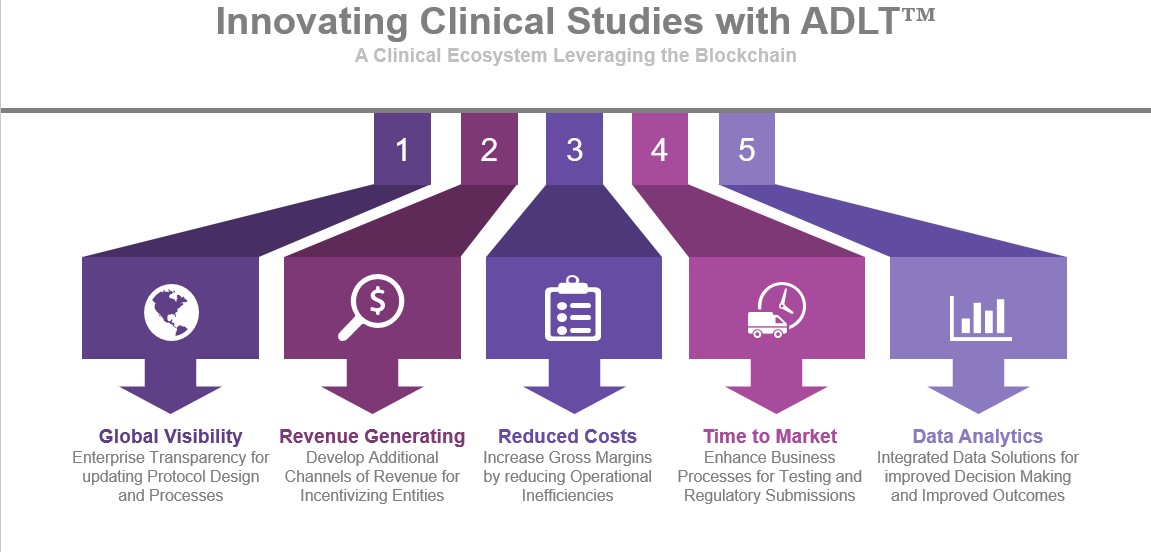
Although the challenges within clinical trials have to do with the unification of clinical trial applications; regulatory readiness; time-to-market and increasing operational costs; one can make an argument that with all the systems and applications supporting a clinical trial, another technology solution will not resolve the existing issues for the challenges created have more to do with policy, procedure and human intervention. However, if we take a deeper look at a specific part of the trial, there are even greater challenges which compound the problem even more. Within Study Startup, Sponsors are challenged by Site Contracting and Budgeting; Site Selection and Identification; Study Planning during Protocol Design and Country Planning/Preparations. How a Blockchain solution solves all these different challenges remains to be seen; however, there is one area within Study Startup whereby the Blockchain can add tremendous value. Which currently would result in the greatest value to the Sponsor! This area is Site Contracting and Budgeting and Protocol Design.
As Site Selection for clinical trial depends upon many factors, there are certain parameters within each Site Contract that the Sponsor can negotiate independently. Moreover, there may be updates to the Protocol Design which may be universal to all sites involved in the trial. The Sponsor needs to have the ability to manage all these moving parts within a timely manner while maintaining a level of transparency and privacy that is not only necessary but also beneficial to the overall success of the trial. As certain costs are transparent to sites via the Charge Master, not every site will contractually be compensated at the same rate as another site. This information should remain private to the Sponsor as no two sites need to know contractually each other’s rate of compensation. However, given that the Sponsor has identified and enrolled the sites, the ability to update and inform the sites of changes and modifications to protocols is essential for allowing the sites to have a uniform level of process and testing. By leveraging ADLT™ by iSolve, Sponsors will be able to manage this part of the trial with a level of visibility, transparency, and privacy that is needed for all parties to act and respond in a manner which provides efficiency in terms of time, costs, negotiations, and communication. Even though more than 90% of the sites are still managing trials via spreadsheets, ADLT™ will allow the Sponsor to manage the communication and process of the trial at an enterprise level while leveraging Blockchain innovation for decentralization and provenance thus helping to reduce the time for Study Startup.
If your organization is involved with a clinical trial and would like to understand how ADLT™ and iSolve can help support your clinical study leveraging the Blockchain, contact us: support@isolve.io. Thanks and we look forward to hearing from you.